Water Properties and Measurements
"... and apportioned the waters by measure,(Job 28:25)
Doctors use instruments like thermometers and stethoscopes to check on your health. Scientists use instruments like Secchi (sek’-ee) disks, probes, nets, gauges, and meters to determine how healthy the water is. They take measurements of the physical and chemical condition of the water and the health of the critters that live in it.
Scientists collect water in lots of different ways. They use boats to go out in the middle of lakes, they wade into streams wearing rubber boots that go up to their chests, they drop buckets over the sides of bridges—they’ll do almost anything to get a sample.
Water samples aren’t the only things scientists collect. They take photographs from airplanes and even satellites. They use their eyes to observe what’s happening along streams, lakes, and bays to get an overall sense of the health of the water. They also collect fish, plants, dirt, and aquatic bugs, and study what’s happening on the land that’s next to the water.
What do scientists measure?
Temperature - When you don’t feel well, chances are the first thing someone does is take your temperature. Scientists measure water temperature for several reasons. First, it determines the kinds of animals that can survive in a stream. If the temperature gets too hot or too cold for some organisms, they die. Temperature also can affect the chemistry of the water. For example, warm water holds less oxygen than cold water. A healthy cluster of trees and vegetation next to a stream or river helps keep temperatures cool for trout and other fish.

Dissolved oxygen probe
Dissolved oxygen - Scientists measure dissolved oxygen, or DO (pronounced dee-oh). This tells them how much oxygen is available in the water for fish and other aquatic organisms to breathe. Healthy waters generally have high levels of DO (some areas, like swamps, naturally have low levels of DO). Just like human beings, aquatic life needs oxygen to survive. Several factors can affect how much DO is in the water. These include temperature, the amount and speed of flowing water, the plants and algae that produce oxygen during the day and take it back in at night, pollution in the water, and the composition of the stream bottom. (Gravelly or rocky bottoms stir up the water more than muddy ones do, creating bubbles that put more oxygen into the water.)
pH - Scientists measure pH to determine the concentration of hydrogen in the water (The p stands for “potential of” and the H is hydrogen.) pH ranges from 0 (very acidic) to 14 (very basic), with 7 being neutral. Most waters range from 6.5 to 8.5. Changes in pH can affect how chemicals dissolve in the water and whether organisms are affected by them. High acidity can be deadly to fish and other aquatic organisms.
The U.S. Geological Survey has been measuring water for decades. Millions of measurements and analyses have been made. Some measurements are taken almost every time water is sampled and investigated, no matter where in the U.S. the water is being studied. Even these simple measurements can sometimes reveal something important about the water and the environment around it.
The results of a single measurement of a water's properties are actually less important than looking at how the properties vary over time. For example, if you take the pH of the creek behind your school and find that it is 5.5, you might say "Wow, this water is acidic!" But, a pH of 5.5 might be "normal" for that creek. It is similar to how my normal body temperature (when I'm not sick) is about 97.5 degrees, but my third-grader's normal temperature is "really normal" -- right on the 98.6 mark. As with our temperatures, if the pH of your creek begins to change, then you might suspect that something is going on somewhere that is affecting the water, and possibly, the water quality. So, often, the changes in water measurements are more important than the actual measured values.
pH is only one measurement of a water body's health; there are others, too. Choose from this list to find out what they are and how they can reveal something about water.
Specific conductance is a measure of the ability of water to conduct an electrical current. It is highly dependent on the amount of dissolved solids (such as salt) in the water. Pure water, such as distilled water, will have a very low specific conductance, and sea water will have a high specific conductance. Rainwater often dissolves airborne gasses and airborne dust while it is in the air, and thus often has a higher specific conductance than distilled water. Specific conductance is an important water-quality measurement because it gives a good idea of the amount of dissolved material in the water.
High specific conductance indicates high dissolved-solids concentration; dissolved solids can affect the suitability of water for domestic, industrial, and agricultural uses. At higher levels, drinking water may have an unpleasant taste or odor or may even cause gastrointestinal distress. Additionally, high dissolved-solids concentration can cause deterioration of plumbing fixtures and appliances. Relatively expensive water-treatment processes, such as reverse osmosis, are needed to remove excessive dissolved solids from water.
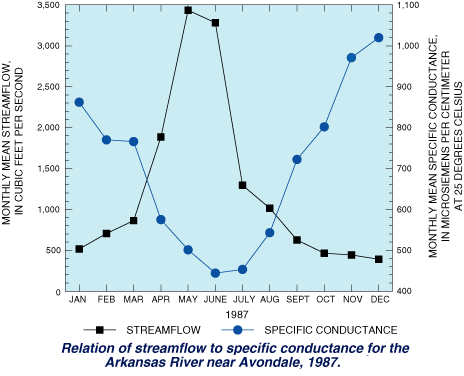
Agriculture also can be adversely affected by high-specific-conductance water, as crops cannot survive if the water they use is too saline, for instance. Agriculture can also be the cause of increases in the specific conductance of local waters. When water is used for irrigation, part of the water evaporates or is consumed by plants, concentrating the original amount of dissolved solids in less water; thus, the dissolved-solids concentration and the specific conductance in the remaining water is increased. The remaining higher specific-conductance water reenters the river as irrigation-return flow. In a USGS study in Colorado, USA, specific conductance was found to vary during the year as a result of the temporal variability of streamflow. As this chart shows, specific conductance generally was lowest in the Arkansas RIver near Avondale, Colorado, in May to August, when streamflow generally was largest, and increased with decreasing streamflow in the fall, winter, and spring.
Often in school, students do an experiment where they connect a battery to a light bulb and run two wires from the battery into a beaker of water. When the wires are put into a beaker of distilled water, the light will not light. But, the bulb does light up when the beaker contains salt water (saline). In the saline water, the salt has dissolved, releasing free electrons, and the water will conduct an electrical current.
Turbidity

Turbidity is the amount of particulate matter that is suspended in water. Turbidity measures the scattering effect that suspended solids have on light: the higher the intensity of scattered light, the higher the turbidity. Material that causes water to be turbid include:
clay
silt
finely divided organic and inorganic matter
soluble colored organic compounds
plankton
microscopic organisms

Turbidity makes the water cloudy or opaque. The picture to the left shows highly turbid water from a tributary (where construction was probably taking place) flowing into the less turbid water of the Chattahoochee River in Georgia. Turbidity is measured by shining a light through the water and is reported in nephelometric turbidity units (NTU). During periods of low flow (base flow), many rivers are a clear green color, and turbidities are low, usually less than 10 NTU. During a rainstorm, particles from the surrounding land are washed into the river making the water a muddy brown color, indicating water that has higher turbidity values. Also, during high flows, water velocities are faster and water volumes are higher, which can more easily stir up and suspend material from the stream bed, causing higher turbidities.


Turbidity can be measured in the laboratory and also on-site in the river. A handheld turbidity meter (left-side picture) measures turbidity of a water sample. The meter is calibrated using standard samples from the meter manufacturer. The picture with the three glass vials shows turbidity standards of 5, 50, and 500 NTUs. Once the meter is calibrated to correctly read these standards, the turbidity of a water sample can be taken.


State-of-the-art turbidity meters (left-side picture) are beginning to be installed in rivers to provide an instantaneous turbidity reading. The right-side picture shows a closeup of the meter. The large tube is the turbidity sensor; it reads turbidity in the river by shining a light into the water and reading how much light is reflected back to the sensor. The smaller tube contains a conductivity sensor to measure electrical conductance of the water, which is strongly influenced by dissolved solids (the two holes) and a temperature gauge (the metal rod).
Dissolved oxygen

You can't tell by looking at water that there is oxygen in it (unless you remember that chemical makeup of a water molecule is hydrogen and oxygen). But, if you look at a closed bottle of a soft drink, you don't see the carbon dioxide dissolved in that - until you shake it up and open the top. The oxygen dissolved in lakes, rivers, and oceans is crucial for the organisms and creatures living in it. As the amount of dissolved oxygen drops below normal levels in water bodies, the water quality is harmed and creatures begin to die off. Indeed, a water body can "die", a process called eutrophication.

Although water molecules contain an oxygen atom, this oxygen is not what is needed by aquatic organisms living in our natural waters. A small amount of oxygen, up to about ten molecules of oxygen per million of water, is actually dissolved in water. This dissolved oxygen is breathed by fish and zooplankton and is needed by them to survive.
Rapidly moving water, such as in a mountain stream or large river, tends to contain a lot of dissolved oxygen, while stagnant water contains little. Bacteria in water can consume oxygen as organic matter decays. Thus, excess organic material in our lakes and rivers can cause an oxygen-deficient situation to occur. Aquatic life can have a hard time in stagnant water that has a lot of rotting, organic material in it, especially in summer, when dissolved-oxygen levels are at a seasonal low.
Hardness

The amount of dissolved calcium and magnesium in water determines its "hardness." Water hardness varies throughout the United States. If you live in an area where the water is "soft," then you may never have even heard of water hardness. But, if you live in Florida, New Mexico, Arizona, Utah, Wyoming, Nebraska, South Dakota, Iowa, Wisconsin, or Indiana, where the water is relatively hard, you may notice that it is difficult to get a lather up when washing your hands or clothes. And, industries in your area might have to spend money to soften their water, as hard water can damage equipment. Hard water can even shorten the life of fabrics and clothes! Does this mean that students who live in areas with hard water keep up with the latest fashions since their clothes wear out faster?



Comments
Post a Comment